(单词翻译:单击)
Since gases are mostly empty space, the densities of gases are reported in g/L, not g/mL as found for solids and liquids. As you’re probably aware, density is equal to mass per unit of volume. To calculate the density of a gas at standard temperature and pressure, you take the molecular formula weight of the gas (grams per mole—from the periodic table) and divide that by thestandard molar volume for a gas, which is 22.4 L per mole: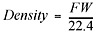
where the formula weight (FW) is in g/mol, and the standard molar volume is 22.4 L/mol. Now try using this in a problem.
Example
What is the density of helium gas at STP?
Explanation
If the density of the gas is equal to 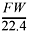 , then d = 4.00 g/mol ∏ 22.4 L/mol, so the density = 0.179 g/L.
, then d = 4.00 g/mol ∏ 22.4 L/mol, so the density = 0.179 g/L.
If conditions are not standard, we can use this expanded version of the ideal gas equation:
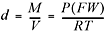
Another really handy rearrangement of the ideal gas equation can be used to find the molecular weight of an unknown gas 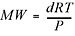 . You’ll get a chance to practice using these in the problems at the end of the chapter. However, there is no need to memorize these last equations since they are all rearrangements of the ideal gas law. Okay, two more important laws and then we’re finished with our discussion of gases, and we move on to solutions.
. You’ll get a chance to practice using these in the problems at the end of the chapter. However, there is no need to memorize these last equations since they are all rearrangements of the ideal gas law. Okay, two more important laws and then we’re finished with our discussion of gases, and we move on to solutions.
以上就是SAT2化学知识讲解:Density of Gases的详细内容,考生可针对文中介绍的方法进行有针对性的备考。


