(单词翻译:单击)
下面SAT频道为大家整理了SAT2化学知识讲解:Chemical Yields,供考生们参考,以下是详细内容。
There are three types of yields you’ll need to be familiar with for the SAT II Chemistry test: theoretical yields, actual yields, and percent yields. Here’s a quick review of what all of these mean to you. The theoretical yield of a reaction is the amount of product formed once the limiting reactant has been completely consumed. This assumes perfect conditions and gives a maximum amount. The actual yield is what actually occurs in the course of the reaction—how much product is actually formed. Finally, the percent yield is the ratio of the actual yield to the theoretical yield, and you can get this value using the following formula:
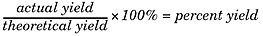
Going back to our last example, the 28,116 grams you calculated would be the theoretical yield. But what would the percent yield be if you performed this reaction and you actually collected 26,450 grams of ammonia? You would calculate it using the above formula. Here it is with the numbers plugged in:
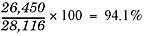
以上就是SAT2化学知识讲解:Chemical Yields的详细内容,考生可针对文中介绍的方法进行有针对性的备考。


