(单词翻译:单击)
France has acted to suspend the sale of a vitamin D supplement after the death of a newborn baby who suffocated hours after being given it.
近日,因发生婴儿在摄入维生素D补充剂几小时后死亡,法国目前已经暂停其销售。
The 10-day-old baby had been given a dose of Uvesterol D, widely given to French children under the age of five to prevent vitamin D deficiency.
这个出生10天的婴儿服用的是一定剂量的Uvesterol D,该药物被广泛应用于法国5岁以下的儿童,以预防维生素D缺乏。
France's medical safety agency said there was a "probable link" to that particular supplement.
据法国的医疗安全机构表示,死亡“可能”和该补充剂“有关”。
Health Minister Marisol Touraine said children were not in danger by taking vitamin D supplements in general as "it's the specific way the product is administered that poses risks". She promised parents "transparent, objective and reliable information."
卫生部长玛丽索尔·杜函娜表示,一般来说,儿童摄入合适量的维生素D并不会有任何危险,他们怀疑可能是通过吸管补充维生素D的方式构成了危险。她向婴儿的父母承诺,会提供“透明、客观和可靠的信息”。
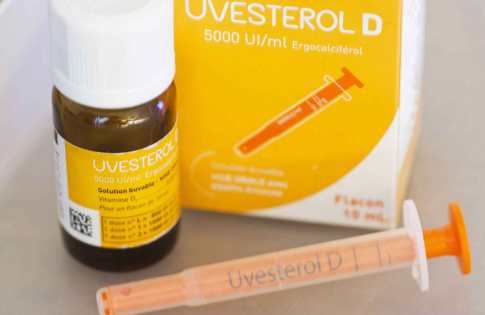
News of the baby's death was not disclosed by France's health authorities immediately but emerged in French media.
该婴儿死亡的消息并没有及时被法国卫生当局披露,而是由法国媒体曝光的。
ANSM said that in 2006 it had imposed measures to reduce risks from taking Uvesterol D after adverse effects became known. However, until December there had been no deaths since it went on the market in 1990, it added.
法国卫生局称,在得知Uvesterol D的副作用后,他们已于2006年采取措施减少其风险。然而,直到去年十二月,自1990年上市以来,该药物没有致死案例。
In 2013, the medical safety agency urged parents to give the supplement drip-by-drip before feeding and ensure the baby was in a semi-sitting position. It also reduced the recommended dosage.
在2013年,医疗安全机构督促父母在使用滴管喂儿童时要确保儿童处于半坐姿,而且需要减少推荐剂量。
In 2014, health journal Prescire called for an end to the use of Uvesterol vitamin supplements for newborn babies, complaining of half-measures and procrastination from both the company and the medical safety agency.
2014年,健康杂志《Prescire》呼吁停止让新生儿服用Uvesterol维生素补充剂,并指责该医药厂商和医疗安全机构没有给予足够措施并拖延解决问题。


