(单词翻译:单击)
Most atoms don't ride solo, instead they bond with other atoms.
大部分的原子不单独行动,它们会跟其他原子发生键合。
And bonds can form between atoms of the same element or atoms of different elements.
相同的原子间可以成键,不同的原子也可以成键。
You've probably imagined bonding as a tug of war.
你可能会把成键的过程想象成拔河。
If one atom is really strong, it can pull one or more electrons off another atom.
如果一个原子很强大,它就可以从另一个原子那里拉拢来一个或者多个电子。
Then you end up with one negatively charged ion and one positively charged ion.
这就能够得到一个带负点荷的离子和一个带正电荷的离子。
And the attraction between these opposite charges is called an ionic bond.
这种相反电荷间的吸引作用就叫做离子键。
This is the kind of sharing where you just give away your toy to someone else and then never get it back.
这种分配就类似于你把你的玩具给了别人,就再也拿不回来了。
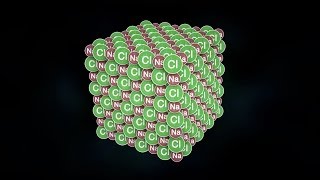
Table salt, sodium chloride, is held together by ionic bonds.
食盐,也就是氯化钠,就是通过离子键形成的。
Every atom of sodium gives up one electron to every atom of chlorine, ions are formed,
每一个钠原子将一个电子提供给一个氯原子,就形成了离子,
and those ions arrange themselves in a 3D grid called a lattice,
这些离子排列形成了一个三维的格子,叫做晶格,
in which every sodium ion is bonded to six chloride ions, and every chloride ion is bonded to six sodium ions.
其中每一个钠离子跟6个氯离子成键,每个氯离子又会跟6个钠离子成键。
The chlorine atoms never give the sodium atoms their electrons back.
氯原子永远不会将电子送返给钠原子。
Now, these transactions aren't always so cut-and-dried.
不过事实上,这种交易不总是这么清晰明朗。
If one atom doesn't completely overwhelm the other, they can actually share each other's electrons.
如果一个原子的吸电子能力并不是完全凌驾于另一个原子之上,它们彼此之间就可以共用电子。
This is like a pot luck where you and a friend each bring a dish and then both of you share both dishes.
这就像在家请客吃饭,你和朋友都准备了各自的菜肴,这样你们就可以分享彼此的美食了。
Each atom is attracted to the shared electrons in between them, and this attraction is called a covalent bond.
每个原子都被共用的电子吸引,这种吸引作用就叫做共价键。
The proteins and DNA in our bodies, for example, are held together largely by these covalent bonds.
打个比方,我们身体里的蛋白质和DNA就是被共价键强力结合起来的。
Some atoms can covalently bond with just one other atom, others with many more.
一些原子只能与单独另一个原子形成共价键,而有很多原子可以形成多个共价键。
The number of other atoms one atom can bond with depends on how its electrons are arranged.
一个原子可以与多少其他原子发生共价结合作用取决于这些电子是如何排布的。
So, how are electrons arranged?
那么,这些电子到底是怎么排布的呢?
Every atom of a pure, unbonded element is electrically neutral
一种单纯的,未发生键合的元素的原子都是不带电荷的,
because it contains the same number of protons in the nucleus as it does electrons around the nucleus.
因为这种原子的原子核携带了与核外电子数目相同的质子。
And not all of those electrons are available for bonding.
不过不是所有的电子都可以成键。
Only the outermost electrons, the ones in orbitals furthest from the nucleus,
只有在最外层,在相距原子核最远的轨道上的电子,
the ones with the most energy, only those participate in bonding.
携带的能量最多,只有它们才能成键。
By the way, this applies to ionic bonding too. Remember sodium chloride?
不仅如此,离子键也是这种情况。还记得氯化钠吗?
Well, the electron that sodium loses is the one furthest from its nucleus,
钠原子失掉的电子也是离核最远的电子,
and the orbital that electron occupies when it goes over to chlorine is also the one furthest from its nucleus.
电子移向氯原子时所占据的轨道也是离核最远的。
But back to covalent bonding. Carbon has four electrons that are free to bond, nitrogen has three, oxygen two.
不过再回头来看共价键。碳原子有4个电子可以自由成键,氮原子有3个电子,氧原子有2个。
So, carbon is likely to form four bonds, nitrogen three, and oxygen two.
所以,碳原子可以形成4个键,氮原子可以形成3个,氧原子能形成2个。
Hydrogen only has one electron, so it can only form one bond.
氢原子只有1个电子,所以只能形成1个键。
In some special cases, atoms can form more bonds than you'd expect,
在某些特殊情况下,原子可以违反常态的形成更多的键,
but they better have a really good reason to do so, or things tend to fly apart.
不过通常都有些特殊的原因,或者物质发生了分解。
Groups of atoms that share electrons covalently with each other are called molecules. They can be small.
彼此间共用电子的一组原子被称作分子。分子可以很小。
For example, every molecule of oxygen gas is made up of just two oxygen atoms bonded to each other.
比方说,每一个氧气分子都是通过2个氧原子键合形成的。
Or they could be really, really big.
分子也可以非常非常大。
Human chromosome 13 is just two molecules, but each one has over 37 billion atoms.
人类基因组13号只含有2个分子,不过每个分子都含有370亿个原子。
And this neighborhood, this city of atoms, is held together by the humble chemical bond.
这种聚合体,庞大的原子群,就是由不起眼儿的化学键连接形成的。


