(单词翻译:单击)
You probably know that all stuff is made up of atoms and that an atom is a really, really, really, really tiny particle.
你或许知道所有东西都是由原子组成的,而原子本身是一个非常非常非常小的粒子。
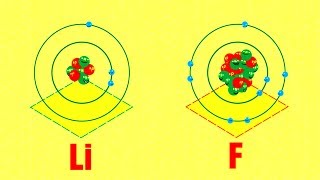
Every atom has a core, which is made up of at least one positively charged particle called a proton,
每个原子都有一个核,由至少一个带正电的粒子组成,这个正电粒子叫做质子,
and in most cases, some number of neutral particles called neutrons.
大多数情况下,还有一些中性粒子叫中子。
That core is surrounded by negatively charged particles called electrons.
原子核被称为电子的负电粒子包围。
The identity of an atom is determined only by the number of protons in its nucleus.
一个原子的特性仅由原子核中质子的数量来确定。
Hydrogen is hydrogen because it has just one proton,
氢就是氢,因为它只有一个质子,
carbon is carbon because it has six, gold is gold because it has 79, and so on.
碳之所以是碳是因为它有6个质子,金之所以是金是因为它有79个质子,诸如此类的还有很多。
Indulge me in a momentary tangent. How do we know about atomic structure?
请允许我讲会儿题外话。我们是如何知道原子的结构的呢?
We can't see protons, neutrons, or electrons.
我们又看不见质子、中子或电子。
So, we do a bunch of experiments and develop a model for what we think is there.
所以,我们做了一堆实验,然后构造一个我们认为是对的模型。
Then we do some more experiments and see if they agree with the model.
然后我们做更多的实验来看看实际情况是否符合模型。
If they do, great. If they don't, it might be time for a new model.
如果是,那最好了。如果不是,那就是时候来建一个新模型了。
We've had lots of very different models for atoms since Democritus in 400 BC,
自公元前400年的德谟克利特以来,我们有很多不同的原子模型,
and there will almost certainly be many more to come. Okay, tangent over.
而且将来肯定会有更多的模型出现。好了,题外话结束。
The cores of atoms tend to stick together,
原子的核心往往是粘在一起的,
but electrons are free to move, and this is why chemists love electrons.
但电子可以自由移动,这就是化学家爱电子的原因了。
If we could marry them, we probably would. But electrons are weird.
如果我们可以和它们结婚,我想我们会的。但是电子很奇怪。
They appear to behave either as particles, like little baseballs,
它们的表现既像是粒子,像是小棒球,
or as waves, like water waves, depending on the experiment that we perform.
也像是波浪,水的波浪,它们在不同的实验中有着不同的表现。
One of the weirdest things about electrons is that we can't exactly say where they are.
关于电子最奇怪的事情是我们无法知道它们的确切位置。
It's not that we don't have the equipment, it's that this uncertainty is part of our model of the electron.
这并不是说我们没有合适的设备,而是这种不确定性也是我们的电子模型的一部分。
So, we can't pinpoint them, fine.
所以,好吧,我们不能精确定位它们。
But we can say there's a certain probability of finding an electron in a given space around the nucleus.
但我们可以说,在原子核周围的一个给定区域中找到一个电子的几率是多少。
And that means that we can ask the following question:
这就意味着我们可以问以下的问题:
If we drew a shape around the nucleus such that we would be 95% sure of finding a given electron within that shape,
如果我们绕原子核画一个形状,使得我们将有95%的把握在这个形状中找到一个给定的电子,
what would it look like?
它会是什么样的?
Here are a few of these shapes.
这里有几个形状。
Chemists call them orbitals, and what each one looks like depends on, among other things, how much energy it has.
化学家们称之为轨道,决定每个轨道的形状的因素之一是它们所拥有能量的多少。
The more energy an orbital has, the farther most of its density is from the nucleus.
一个轨道拥有的能量越多,那么它的主要部分就离原子核越远。
By they way, why did we pick 95% and not 100%?
顺便提一下,为什么我们选择95%的把握而不是100%?
Well, that's another quirk of our model of the electron.
好吧,那是我们电子模型另一个比较特殊的地方。
Past a certain distance from the nucleus,
从离开原子核的一定距离开始,
the probability of finding an electron starts to decrease more or less exponentially,
发现电子的几率开始随着距离下降,差不多呈现指数衰减,
which means that while it will approach zero, it'll never actually hit zero.
这就意味着几率会越来越接近零,但事实上永远不会达到零。
So, in every atom, there is some small, but non-zero, probability that for a very, very short period of time,
所以,每一个原子中,总有一些很小但是非零的可能性,在很短很短的一段时间里,
one of its electrons is at the other end of the known universe.
其中一个电子正位于宇宙的另一端。
But mostly electrons stay close to their nucleus as clouds of negative charged density that shift and move with time.
但是大部分时间电子都距离原子核很近,呈现为带负电的电子云,电子云会随着时间改变位置。
How electrons from one atom interact with electrons from another determines almost everything.
一个原子的电子如何和另一个原子的电子互相作用几乎决定了一切。
Atoms can give up their electrons, surrendering them to other atoms, or they can share electrons.
原子可以放弃自己的电子,把它们给其他原子,或者与其他原子共享电子。
And the dynamics of this social network are what make chemistry interesting.
而这个社交网络的动态使化学变得有趣。
From plain old rocks to the beautiful complexity of life,
从普通的旧石头到美好而复杂的生命,
the nature of everything we see, hear, smell, taste, touch, and even feel is determined at the atomic level.
自然界中一切我们可以看到的、听到的、闻到的、品尝到的、触摸到的,甚至是能感觉到的,都是在原子层面决定的。


