(单词翻译:单击)
In 1928, chemists invented a type of molecule that seemed perfect.
1928年,化学家发明了一种似乎完美的分子.
By the seventies, these molecules were in all kinds of things,
到了70年代,这些分子被用作各种东西,
making companies billions of dollars a year.
让工厂一年赚数十亿美元。
Then, in 1974, a pair of scientists discovered that
随后在1974年,两位科学家发现
these miracle molecules were destroying a part of Earth's atmosphere that keeps us alive.
这种神奇的分子正在破坏我们赖以生存的地球大气。
It took years to convince the world they were right,
他们用了数年的时间让人们相信他们是对的,
and another three decades for the planet to finally start healing.
又花了三十年的时间地球才最终开始好转。
But these scientists didn't even set out to save the Earth,
但这些科学家们没有开始拯救地球,
they were just studying what happens on Venus.
他们只是研究金星的状况。
Those seemingly perfect molecules were chlorofluorocarbons, or CFCs,
那些看似完美的分子是氯氟烃(CFCs),
which have chlorine, fluorine, and carbon atoms bonded together in different ways.
是氯原子、氟原子和碳原子以不同方式结合的产物。
The great thing about CFCs was that almost nothing would react with them or break them apart.
氯氟烃的优点是它几乎不与其他物质反应,也不会被分解。
The carbon-fluorine bonds in a CFC like Teflon, for example, are so strong that the strongest acids in the world can't break them.
氯氟烃的碳氟键像聚四氟乙烯,非常牢固,以至于世界上最强的酸也破坏不了。
Plus, CFCs were non-toxic, fireproof, and relatively easy to make for lots of different purposes.
而且,氯氟烃无毒、耐火,用于不同用途相对容易。
So CFCs were all over the place by the seventies:
所以到了70年代,氯氟烃到处都是:
refrigerators, fire extinguishers, aerosols, solvents, insulation, you name it.
冰箱、灭火器、喷雾器、溶剂、绝缘体等,应有尽有。
A million metric tons of gaseous CFCs were released into the atmosphere every year,
每年一百万公吨的氯氟烃气体被释放到大气中,
and at the time scientists just assumed that
而当时的科学家认为
CFCs would keep being their wonderfully inert selves in the atmosphere and not cause any problems.
氯氟烃会在大气中保持自己良好的惰性,不会造成任何麻烦。
CFCs were so unreactive down here at the surface
地球表面的氯氟烃没反应,
that scientists working on them didn't bother checking what happened when they got higher up.
以至于科学家们没有麻烦地检查它们到高处时的情况。
So they missed something important:
所以他们错过了重要的事:
CFCs were destroying the ozone layer, the part of Earth's atmosphere
氯氟烃正破坏地球大气中的臭氧层,
made up of three-atom oxygen molecules that absorb a lot of harmful UV radiation before it reaches the surface.
臭氧层是由三个氧原子构成的,能吸收大量的到达地球表面的有害辐射。
And it took researchers working on a completely different planet-wide conundrum, on a totally different planet to figure that out.
通过对另一个不同行星的难题的研究,研究人员找到了这个问题的答案。
Venus's atmosphere is mostly carbon dioxide,
金星的大气层主要是二氧化碳,
with a tiny bit of nitrogen, water vapor, carbon monoxide, and hydrochloric, hydrofluoric, and sulfuric acids.
也有少量的氮、水蒸气、一氧化碳、盐酸、氢氟酸和硫酸。
Basically, it's not somewhere you'd wanna go on vacation.
它基本上是你不想度假的地方。
All that carbon dioxide confused scientists in the early 1970s,
它上面的二氧化碳在19世纪70年代早期让科学家们感到困惑,
because they didn't see an ozone layer on Venus.
因为他们在金星上没有看到任何臭氧层。
UV light breaks molecules apart, and they knew that without ozone to block it,
紫外线破坏分子,他们知道没有臭氧的阻止,
UV light from the sun should've broken apart a lot of Venus's CO2,
来自太阳的紫外线应该会破坏金星上大量的二氧化碳,
leaving behind much more oxygen and carbon monoxide than they were seeing.
留下的氧气和一氧化碳比他们看到的应该多很多。
Then, groups led by environmental scientist Michael McElroy figured out what was probably happening:
随后, 环境学家麦克尔·罗伊带领的小组弄清了上面的情况:
The same UV light that would break down CO2 would break down hydrochloric acid at the same time, freeing up hydrogen atoms.
分解二氧化碳的紫外线同时也会分解盐酸,释放出氢原子。
Then, those hydrogen atoms would act like ruthless matchmakers,
然后这些氢原子会充当无情的媒人,
ripping oxygen atoms off of molecules so that they combined with carbon monoxide to make carbon dioxide again.
撷取分子中的氧原子,使它们与一氧化碳结合,再次形成二氧化碳。
That's why they saw so much carbon dioxide in Venus's atmosphere,
这就是科学家在金星大气中看到那么多二氧化碳的原因,
even though it wasn't being protected by an ozone layer.
即使它没有臭氧层的保护。
When UV radiation broke apart CO2, a small army of hydrogen atoms would just put it back together again.
当紫外线辐射使二氧化碳分解后,一小队氢原子会再将它重新组装回来。
It took decades to prove that this was actually happening in the Venusian skies,
科学家们花了几十年才证明金星大气中发生的事,
along with a similar process that involved chlorine instead of hydrogen.
又发现了一个类似的氯取代氢的现象。
But in the meantime, a pair of chemists named Mario Molina and Sherwood Rowland realized
但与此同时,两名叫马里奥·莫利纳和舍伍德·罗兰的化学家意识到
that something similar was happening on Earth, too, which meant we had a major problem.
类似的事也在地球上上演,这意味着我们麻烦大了。
CFCs might be totally unreactive down here on the surface,
氯氟烃可能在地球表面全无反应,
but they realized that would change once the molecules reached the upper atmosphere
但他们意识到一旦分子到达上层大气就会发生变化,
and got bombarded by that powerful UV radiation.
会被强有力的紫外线辐射轰炸。
The UV could break CFCs apart and leave chlorine atoms free to rip oxygen off of anything nearby,
紫外线能使氯氟烃分解,留下自由的氯原子撷取附近的氧气,
just like hydrogen does on Venus when it's putting carbon dioxide back together.
这和金星上的氢组合二氧化碳时做得一样。
So all those CFCs in the upper atmosphere would lead to a lot of chlorine stealing oxygen atoms from ozone molecules,
因此,上层大气中的所有氯氟烃会留下大量的氯原子盗取臭氧分子中的氧原子,
which meant that there were fewer ozone molecules around to block UV light.
也就是说阻挡紫外线辐射的臭氧分子变少了。
And a single chlorine atom could tear apart a hundred thousand ozone molecules before permanently bonding to something.
一个氯原子在臭氧分子与某物结合前能够破坏10万个臭氧分子。
So Molina and Rowland were saying that those supposedly unreactive CFCs were eating away at the ozone layer
所以莫利纳和罗兰说这种可能惰性的氯氟烃正在蚕食臭氧层,
that protects us from things like skin cancer, and protects the entire world from widespread crop failures.
而臭氧层能保护我们不患皮肤癌,保护整个世界的农作物。
But everyone making CFCs wasn't just going to stop because a couple chemists said so.
但制造氯氟烃的人们不会因为几个化学家的言辞就停止生产。
Molina and Rowland were studying Venus, not Earth.
莫利纳和罗兰研究的是金星,而不是地球。
And besides, everyone knew that CFCs didn't react with anything.
而且,每个人都知道氯氟烃不与任何物质反应。
That's what made them so great!
这显得它们如此伟大!
But soon, the evidence started piling up.
但不久,证据开始大量出现。
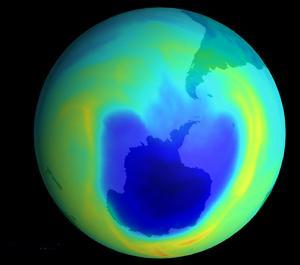
In 1985, scientists discovered a hole in the ozone layer above Antarctica that was getting bigger every year.
1985年,科学家们发现南极洲上空的一个臭氧层洞一年比一年大。
Some people were still skeptical, though, until researchers found chlorine and fluorine in the hole itself,
不过有些人仍持怀疑态度,直到研究人员在洞里发现了氯和氟,
and others found that the ozone layer was getting thinner all over the world.
其他人也发现世界的臭氧层正变得越来越薄。
Today, there is no doubt about it: Molina and Rowland were right.
如今,毫无疑问:莫利纳和罗兰是正确的。
And in 1995, they won the Nobel Prize in Chemistry for their discovery.
1995年,他们因此项发现获得了诺贝尔化学奖。
Since the eighties, international agreements have just about stopped CFC production around the world,
自80年代起,全世界签署国际协议停止氯氟烃的生产,
and corporations have been looking for safer alternatives.
企业一直在寻找更安全的替代方案。
So far, they haven't found many good ones,
到目前为止,他们还没有找到太多好的替代品,
and the ones they have found generally still aren't great for the environment.
已经发现的仍对环境不友好。
But at least they're better than CFCs.
但至少比氯氟烃好多了。
And in just the last couple of years, scientists have finally seen the hole over Antarctica start to shrink,
在过去几年,科学家们终于看到南极洲上空的臭氧洞开始收缩了,
more than forty years after a technical mystery in the Venusian skies accidentally saved the planet.
金星上空的技术秘密在40多年后意外拯救了地球。
Thanks for watching this episode of SciShow Space,
感谢您收看本期的太空科学秀,
and thank you especially to our patrons on Patreon who make this show possible.
同时也感谢Patreon对本节目的大力赞助。
If you want to help us keep making episodes like this, you can go to patreon.com/scishow.
如果你想帮我们继续制作此节目,就登录patreon.com/scishow吧。
And don't forget to go to youtube.com/scishowspace and subscribe!
同时不要忘了登录youtube.com/scishowspace点击订阅哦!


