(单词翻译:单击)
Water is the liquid of life.
水是生命的体液。
We drink it, we bathe in it, we farm, cook, and clean with it.
人喝水,用水洗澡、耕种、做饭、清洁。
It's the most abundant molecule in our bodies.
水是人身体中数量最多的分子。
In fact, every life form we know of would die without it.
事实上,如果没有水,所有的生命都会死亡。
But most importantly, without water, we wouldn't have iced tea. Mmmm, iced tea.
但最重要的是,如果没有水,就没有冰茶了。嗯,冰茶。
Why do these ice cubes float?
为什么冰块会浮在上面呢?
If these were cubes of solid argon in a cup of liquid argon, they would sink.
如果这些冰块是固体氩气做成的,被放入一杯液体氩气中,他们就会下沉。
And the same goes for most other substances.
大多数其他物质都是固态重于液态。
But solid water, a.k.a. ice, is somehow less dense than liquid water. How's that possible?
但是不知为何,固态水,又称冰,密度比液态水小。怎么可能呢?
You already know that every water molecule is made up of two hydrogen atoms bonded to one oxygen atom.
众所周知,一个水分子是两个氢原子和一个氧原子组成的。
Let's look at a few of the molecules in a drop of water, and let's say the temperature is 25 degrees Celcius.
请看一滴水中的几个分子,假设温度是25摄氏度。
The molecules are bending, stretching, spinning, and moving through space.
这时,分子在弯曲,伸展,旋转,在空间里移动。
Now, let's lower the temperature, which will reduce the amount of kinetic energy each of these molecules has
现在,降低温度,每个分子里所含的动能也随之减少,
so they'll bend, stretch, spin, and move less.
那么分子弯曲、拉伸、旋转,移动地自然会较少。
And that means that on average, they'll take up less space.
平均而言,这意味着分子所占空间变小。
Now, you'd think that as the liquid water starts to freeze,
那么,你可能以为,液态水在冻结时,
the molecules would just pack together more and more closely, but that's not what happens.
分子只是挤在一起,贴得越来越紧,但事实并非如此。
Water has a special kind of interaction between molecules that most other substances don't have, and it's called a hydrogen bond.
水,同大多数其他物质不同,水分子之间有一种独特的的相互作用叫做氢键。
Now, remember that in a covalent bond two electrons are shared, usually unequally, between atoms.
通常在共价键里,两个电子在原子之间被不平等共享。
In a hydrogen bond, a hydrogen atom is shared, also unequally, between atoms.
在氢键里,氢原子在原子之间也被不平等共享。
One hydrogen bond looks like this. Two look like this.
一个氢键是这样的。两个是这样。
Here's three and four and five, six, seven, eight, nine, ten, eleven, twelve, I could go on.
这是三个、四个,五、六、七、八、九、十、十一、十二,我还可以加下去哦。
In a single drop of water, hydrogen bonds form extended networks
一滴水里,氢键形成大范围网络,
between hundreds, thousands, millions, billions, trillions of molecules, and these bonds are constantly breaking and reforming.
在数百、数千、数以百万,数十亿,数万亿的分子之间,这种连接又不断地断开再重新形成。
Now, back to our water as it cools down.
现在还是回头讲水凉了的情况吧。
Above 4 degrees Celcius, the kinetic energy of the water molecules keeps their interactions with each other short.
4摄氏度以上时,水分子的动能使其之间的互动时间很短。
Hydrogen bonds form and break like high school relationships, that is to say, quickly.
氢键快速形成再断开,就好比是高中生之间的关系,真的是速战速决。
But below 4 degrees, the kinetic energy of the water molecules starts to fall below the energy of the hydrogen bonds.
但温度低于4度时,水分子的动能开始跌落,低于氢键内能源量。
So, hydrogen bonds form much more frequently than they break and beautiful structures start to emerge from the chaos.
所以,氢键形成比断开还频繁,混乱中,开始呈现出新的美妙结构。
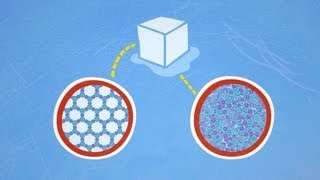
This is what solid water, ice, looks like on the molecular level.
固态水,冰,从分子图上是这样的。
Notice that the ordered, hexagonal structure is less dense than the disordered structure of liquid water.
请注意,这种有序的六角形结构的密度低于无序结构的液态水。
And you know that if an object is less dense than the fluid it's in, it will float.
大家都知道,如果物体放入比其密度低的液体中,该物体就会浮起来。
So, ice floats on water, so what?
好吧,冰浮于水上又有什么了不起的?
Well, let's consider a world without floating ice.
好,设想一下没有浮冰的世界吧。
The coldest part of the ocean would be the pitch-black ocean floor, once frozen, always frozen.
海洋最冷的部分将是漆黑不见五指的海底,一旦冻结就会永远冻结。
Forget lobster rolls since crustaceans would lose their habitats, or sushi since kelp forests wouldn't grow.
别指望再吃到龙虾卷了,因为甲壳类动物就会失去它们的栖息地,寿司也永别了,因为海藻森林不会增长。
What would Canadian kids do in winter without pond hockey or ice fishing?
如果没法玩冰球或冰中钓鱼,那加拿大的孩子们在冬天要做什么?
And forget James Cameron's Oscar because the Titanic totally would have made it.
詹姆斯·卡梅隆也可以忘记他的奥斯卡奖了,因为泰坦尼克号根本没机会沉。
Say goodbye to the white polar ice caps reflecting sunlight that would otherwise bake the planet.
永别了! 那反射着阳光的雪白的极地冰帽,而阳光则烘烤着我们星球。
In fact, forget the oceans as we know them,
事实上,连我们熟知的海洋也将不复存在,
which at over 70% of the Earth's surface area, regulate the atmosphere of the whole planet.
现在地球表面面积的70%以上是海洋,调节着整个地球的大气层。
But worst of all, there would be no iced tea. Mmmmm, iced tea.
但最糟的是,那样就没有冰茶了。嗯,冰茶。


