(单词翻译:单击)
In the early days of organic chemistry, chemists understood that molecules were made of atoms connected through chemical bonds.
在有机化学的早期,化学家们知道了分子是由原子构成的,由化学键相互连接。
However, the three-dimensional shapes of molecules were utterly unclear, since they couldn't be observed directly.
但是,分子的三维形状却鲜为人知,因为他们不能被直接观察到。
Molecules were represented using simple connectivity graphs like the one you see here.
分子用简单的连接图表示,就像你现在看到的这样。
It was clear to savvy chemists of the mid-19th century that these flat representations couldn't explain many of their observations.
19世纪中期,精明的化学家们清楚,这样的扁平模型不能解释他们观察到的很多现象。
But chemical theory hadn't provided a satisfactory explanation for the three-dimensional structures of molecules.
但是化学理论没能提供一个令人满意的解释来说明分子的三维结构。
In 1874, the chemist Van't Hoff published a remarkable hypothesis:
在1874年,化学家范特霍夫发表了这个伟大的假说:
the four bonds of a saturated carbon atom point to the corners of a tetrahedron.
一个饱和碳原子的四根键指向一个四面体的角。
It would take over 25 years for the quantum revolution to theoretically validate his hypothesis.
在超过25年之后,量子革命才从理论上证实了他的假说。
But Van't Hoff supported his theory using optical rotation.
但是范特霍夫用旋光效应来支撑他的理论。
Van't Hoff noticed that only compounds containing a central carbon bound to four different atoms or groups rotated plane-polarized light.
他注意到,只有具有中心碳原子并连接到四个不同原子或原子团的化合物,才能旋转平面偏振光。
Clearly there's something unique about this class of compounds.
很明显,这类化合物有独特的性质。
Take a look at the two molecules you see here.
仔细观察这里的两个分子。
Each one is characterized by a central, tetrahedral carbon atom bound to four different atoms: bromine, chlorine, fluorine, and hydrogen.
每一个都具有四面体形的中心碳原子,分别连接四个不同的原子:溴,氯,氟和氢。
We might be tempted to conclude that the two molecules are the same, if we just concern ourselves with what they're made of.
我们很可能会得出结论,这两个分子是一样的,如果我们只考虑它们的组成成分。
However, let's see if we can overlay the two molecules perfectly to really prove that they're the same.
但是,我们来看看能否通过完全重叠两个分子来证明它们真的是一样的。
We have free license to rotate and translate both of the molecules as we wish.
我们可以随意旋转和翻转这两个分子。
Remarkably though, no matter how we move the molecules, we find that perfect superposition is impossible to achieve.
但值得注意的是,不管我们怎样移动这些分子,完全重叠都是不可能完成的。
Now take a look at your hands. Notice that your two hands have all the same parts: a thumb, fingers, a palm, etc.
现在看看你的手。注意你的两只手有着相同的部分:拇指,手指,手掌等等。
Like our two molecules under study, both of your hands are made of the same stuff.
像我们研究的两个分子一样,你的两只手是同样的成分组成的。
Furthermore, the distances between stuff in both of your hands are the same.
更进一步,你两只手上各部分间的距离也是相同的。
The index finger is next to the middle finger, which is next to the ring finger, etc.
食指临近中指,中指靠着无名指,以此类推。
The same is true of our hypothetical molecules.
我们所假设的分子也是这样的。
All of their internal distances are the same.
它们的所有内部距离都是相同的。
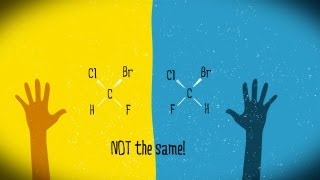
Despite the similarities between them, your hands, and our molecules, are certainly not the same.
尽管它们有很多共同点,你的手和我们的分子显然不是相同的。
Try superimposing your hands on one another.
试试把你的手重叠到一起。
Just like our molecules from before, you'll find that it can't be done perfectly.
就像我们之前的分子一样,你会发现它们不能完全重叠。
Now, point your palms toward one another. Wiggle both of your index fingers.
现在,把你的手掌相对。晃动你的两只食指。
Notice that your left hand looks as if it's looking in a mirror at your right.
你会发现你的左手好像在通过镜子看你的右手。
In other words, your hands are mirror images.
换言之,你的两只手是镜面图像。
The same can be said of our molecules.
我们的分子也是一样的。
We can turn them so that one looks at the other as in a mirror.
我们把它们转过来,所以一个看着另一个,就像镜子里一样。
Your hands - and our molecules - possess a spatial property in common called chirality, or handedness.
你的手,和分子,拥有同样的一种空间特性,叫做手征,也叫手性。
Chirality means exactly what we've just described: a chiral object is not the same as its mirror image.
手性就是指我们刚刚描述过的特性:一个手性物体和它的镜像并不同。
Chiral objects are very special in both chemistry and everyday life.
手性物体在化学和日常生活中都很特殊。
Screws, for example, are also chiral.
螺丝钉,打个比方,就是手性的。
That's why we need the terms right-handed and left-handed screws.
所以我们才需要区分左手和右手螺钉。
And believe it or not, certain types of light can behave like chiral screws.
不管你信不信,某些种类的光也像手性螺丝一样。
Packed into every linear, plane-polarized beam of light are right-handed and left-handed parts
在每一束线性的平面偏振光中都有左手和右手部分
that rotate together to produce plane polarization.
共同旋转来产生平面偏振。
Chiral molecules, placed in a beam of such light, interact differently with the two chiral components.
手性分子,当放在这样的光束中时,对两个手性部分产生不同的反应。
As a result, one component of the light gets temporarily slowed down relative to the other.
作为结果,光的一部分相对另一部分被暂时减缓。
The effect on the light beam is a rotation of its plane from the original one, otherwise known as optical rotation.
对光线的影响就是相对初始平面的平面旋转,也被称作旋光。
Van't Hoff and later chemists realized that the chiral nature of tetrahedral carbons can explain this fascinating phenomenon.
范特霍夫和后来的化学家认识到,四面体碳的手性可以解释这个奇妙的现象。
Chirality is responsible for all kinds of other fascinating effects in chemistry, and everyday life.
手性也导致了其他很多化学和日常生活中的奇妙影响。
Humans tend to love symmetry and so if you look around you, you'll find that chiral objects made by humans are rare.
人类喜欢对称,所以如果你看看周围,你会发现人类制造的手性物体非常罕见。
But chiral molecules are absolutely everywhere.
但是手性分子确实哪里都有。
Phenomena as separate as optical rotation, Screwing together furniture, and clapping your hands all involve this intriguing spatial property.
很多独立的现象类似旋光,钉家具和拍手,都包含着这个引人入胜的空间特性。


