(单词翻译:单击)
OK, today we're going to talk about the mole.
OK,今天我们来谈一下摩尔。
Now, I know what you're thinking: "I know what a mole is, it's a small creature that dig holes in the ground and destroys gardens."
我知道你在想什么:“我知道mole是什么,它是一只小动物,在地面上挖洞,毁坏花园。”
And some of you might be thinking that it's a growth on your aunt's face with hairs sticking out of it.
有些人也许会认为那是长在阿姨脸上,并带有几根细毛的痣。
Well, in this case, a mole is a concept that we use in chemistry to count molecules, atoms, just about anything extremely small.
但今天所讲的摩尔,是化学中的一个概念,用来计算分原子、或是任何极小物质的数量。
Have you ever wondered how many atoms there are in the universe? Or in your body? Or even in a grain of sand?
你曾经想过这个宇宙到底有多少的原子吗?或是你的身体里?或是在一粒沙子里?
Scientists have wanted to answer that question, but how do you count something as small as an atom?
科学家想解开这个问题,但是你要如何计算小到像原子的东西?
Well, in 1811, someone had an idea that if you had equal volumes of gases,
在1811年,有个人突发奇想,认为如果你有相同体积的气体,
at the same temperature and pressure, they would contain an equal number of particles.
在相同的温度及压力下,它们应该会有相同的分子数量,
His name was Lorenzo Romano Amedeo Carlo Avogadro.
他的名字是阿莫迪欧·阿伏伽德罗
I wonder how long it took him to sign autographs.
我好奇他签个名要花多久时间。
Unfortunately for Avogadro, most scientists didn't accept the idea of the atom, and there was no way to prove he was right.
对阿伏伽德罗来说,很不幸的是,大部分的科学家不接受这个想法,而他也没有方法可以证明他是对的。
There was no clear difference between atoms and molecules.
原子和分子之间并没有明显的差异。
Most scientists looked at Avogadro's work as purely hypothetical, and didn't give it much thought.
大多数的科学家认为阿伏伽德罗的理论纯粹是个假说,并没有特别去思考这件事。
But it turned out he was right! By late 1860, Avogadro was proven correct,
但事实证明他是对的!1860年底,阿伏伽德罗的想法被证明是对的,
and his work helped lay the foundation for the atomic theory. Unfortunately, Avogadro died in 1856.
而且他的努力奠定了原子理论的基石。不幸的是,阿伏伽德罗死于1856年。
Now the thing is that the amount of particles in even small samples is tremendous. For example,
问题是,即使是小东西上的分子总数也是非常巨大的。
If you have a balloon of any gas at zero degrees Celcius, and at a pressure of one atmosphere,
假使你有一个汽球,装进任一种气体,在摄氏零度和一大气压的条件下,
then you have precisely six hundred and two sextillion gas particles.
那你会有刚刚好602乘10的21次方个气体分子。
That is, you have six with 23 zeros after it particles of gas in the container.
就是6后面带有23个0,这么多个分子在里面。
Or in scientific notation, 6.02 times10 to the 23rd particles.
或用科学记号表示为6.02乘以10的23次方个分子。
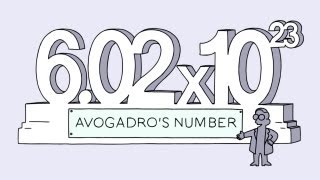
This example is a little misleading, because gases take up a lot of space due to the high kinetic energy of the gas particles,
这个例子有点误导人,因为高动能的气体分子会占用较大的空间,
and it leaves you thinking atoms are bigger than they really are.
这让你感觉原子比实际大小的还要大。
Instead, think of water molecules.
相对地,我们看一下水分子。
If you pour 18.01 grams of water into a glass, which is 18.01 milliliters,
如果你倒入18.01公克的水到杯子里,就是18.01毫升,
which is like three and a half teaspoons of water, you'll have 602 sextillion molecules of water.
大约是三个半茶匙的水,你就有602乘以10的21次方个水分子。
Since Lorenzo Romano - uh, never mind - Avogadro was the first one to come up with this idea,
自从Lorenzo Romano...嗯,算了。阿伏伽德罗是第一位提出这个想法的人,
scientists named the number 6.02 times 10 to the 23rd after him.
科学家用他的名字来命名6.02乘以10的23次方这个数字。
It is simply known as Avogadros's number. Now, back to the mole. Not that mole.
这就是熟知的阿伏伽德罗常数。现在,回到莫尔。不是在说鼹鼠。
This mole. Yep, this number has a second name.
这个摩尔。没错,这个常数有另一个名字。
The mole. Chemists use the term mole to refer to the quantities that are at the magnitude of 602 sextillion.
摩尔,化学家使用莫耳这个术语来表示602乘以10的21次方这个数量。
This is known as a molar quantity.
就是大家知道的摩尔常数。
Atoms and molecules are so small, that chemists have bundled them into groups called moles.
原子和分子太小了,所以化学家将它们分成一群一群,每一群就叫做一摩尔。
Moles are hard for students to understand because they have a hard time picturing the size of a mole, or of 602 sextillion.
摩尔对于学生是很难了解的,因为他们很难想象1 莫耳的数量,或是602乘以10的21次方。
It's just too big to wrap our brains around.
它实在大到大脑难以理解。
Remember our 18.01 milliliters of water? Well, that's a mole of water. But how much is that?
记得刚刚说的18.01毫升的水吗?那就是1摩尔的水。但是这有多少?
Exactly what does 602 sextillion look like?
602乘以10的21次方看起来像什么?
Maybe this'll help. Exchange the water particles for donuts.
也许这有帮助。用甜甜圈来代替水分子。
If you had a mole of donuts, they would cover the entire earth to a depth of eight kilometers, which is about five miles.
假如你有1摩尔的甜甜圈,它们可以覆盖整个地球表面到8公里高,大约是5英里。
You really need a lot of coffee for that.
你需要很多咖啡来配。
If you had a mole of basketballs, you could create a new planet the size of the earth.
如果你有1摩尔的篮球,你可以制造和地球一样大的星球。
If you received a mole of pennies on the day you were born and spent a million dollars a second until the day you died at the age of 100,
如果你一出生时,你有1摩尔的钱,你每一秒花一百万直到100岁你死的那一天,
you would still have more than 99.99% of your money in the bank.
你还有99.99%以上的钱在银行里。
OK.Now we sort of have an idea how large the mole is. So how do we use it?
好了,现在我们大概对1摩尔有个概念了。我们如何用它?
You might be surprised to know that chemists use it the same way you use pounds to buy grapes, deli meat, or eggs.
你也许会惊讶化学家使用它的方法,就如同你用‘磅’来买葡萄,熟食肉类,或鸡蛋。
When you go to the grocery store, you don't go to the deli counter and ask for 43 slices of salami, you buy your salami by the pound.
当你到杂货店时,你不会走到柜台要43片腊肠,你会买几磅的腊肠。
When you buy your eggs, you buy a dozen eggs.
当你买鸡蛋时,你会买一打鸡蛋。
When we hear the word dozen, we probably think of the number 12.
当我们听到‘打’这个字,我们会想到数字 12。
We also know that a pair is two, a baker's dozen is 13, a gross is 144, and a ream of paper is - anybody?
我们也知道一双就是2,面包师父的一打是13,一箩是144,一令纸......谁知道?
A ream is 500. Well, a mole is really the same thing.
一令纸是500张。一摩尔也是一样的。
For a chemist, a mole conjures up the number 6.02 times 10 to the 23rd, not a fuzzy little animal.
对化学家而言,1摩尔就是 6.02乘以10的23次方而不是毛绒绒的动物。
The only difference is that the other quantities are more familiar to us.
唯一的差别是我们比较熟悉其他的计数单位。
So there you have it - the story of the mole, Avogadro, basketballs, and how to buy salami at the grocery store.
现在你知道了摩尔的故事。阿伏伽德罗、篮球、还有去杂货店怎么买腊肠。


